Researchers in the US have created a new solid-state lithium-metal battery that can charge and discharge over a record-breaking number of cycles at a high current density. The proof-of-concept device, which is fundamentally different from existing liquid electrolyte lithium-ion batteries, could extend the lifespan of electric vehicle batteries to 10–15 years, similar to that of petrol and diesel cars.
The ideal battery for an electric vehicle would last a long time and store up a lot of charge very quickly. While lithium-metal batteries score well on the second of these criteria thanks to their high capacity and high energy density, their longevity leaves something to be desired. This is because, during charging, lithium ions move from the cathode to the anode. When this anode is made of lithium metal, needle-like structures called dendrites form on the electrode surface and grow into the electrolyte. Eventually, these unwanted structures pierce the barrier separating the anode and cathode, causing the battery to short or even ignite.
To overcome this problem, researchers have previously tried replacing the liquid electrolyte in these devices with a solid-state one that is more difficult for the dendrites to grow through. In practice, however, lithium dendrites can still burrow through the barrier via micron- or submicron-sized cracks produced when the battery is assembled.
Sandwich effect
Researchers led by Xin Li, a professor of materials science at Harvard University’s John A Paulson School of Engineering and Applied Sciences (SEAS), have now developed a solid-state battery in which lithium dendrite penetration is no longer a problem. Rather than stopping the dendrites dead in their tracks with a single barrier, their new battery design takes a multi-layered approach. The design, which is detailed in Nature, incorporates a less-stable electrolyte sandwiched between layers of more-stable solid electrolytes. Together, these layers keep dendrite growth under control.
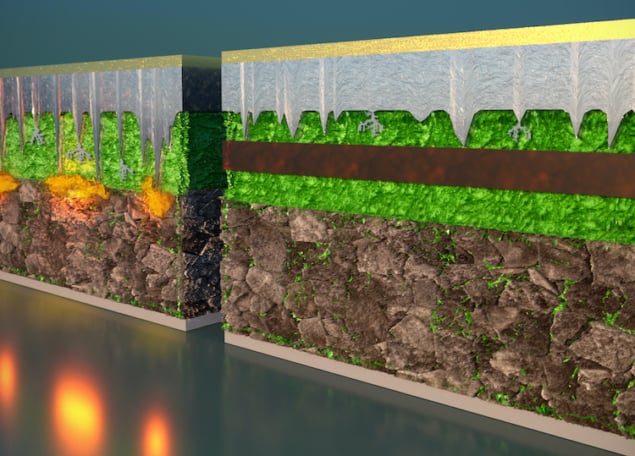
The full battery “sandwich” consists of six layers: a lithium-metal anode, a graphite coating, the first electrolyte, the second electrolyte, another layer of the first electrolyte, and finally the cathode. The first electrolyte, which has the chemical formula Li5.5PS4.5Cl1.5 (LPSCI), is prone to dendrite penetration. The second electrolyte, Li10Ge1P2S12(LGPS), is more robust. The idea is that dendrites are permitted to grow through the graphite and electrolyte no. 1 but stop when they reach electrolyte no. 2 – and, crucially, before they short out the battery.
“Incorporating instability to stabilize the battery might sound counterintuitive,” says team member Luhan Ye. “But just like an (expansion) anchor can guide and control a screw going into a drywall, so too can our multilayer design guide and control the growth of dendrites. The difference is that our anchor (the LPSCI) quickly becomes too tight for the dendrite to drill through, so the dendrite growth is stopped.”
Superior cycling performance
The researchers found that when they paired their lithium metal anode with a LiNi0.8Mn0.1Co0.1O2 cathode, the cycling performance was very stable, with the battery maintaining 82% of its capacity after 10,000 cycles at a 20C rate (8.6 mA/cm2) and 81.3 % of its capacity after 2000 cycles at a 1.5C rate (0.64 mA/cm2). The design allows for a specific power of 110.6 kW/kg and a specific energy of up to 631.1 Wh/kg from the cathode material.
The test device cycled for 1800 hours at 0.25 mA/cm2, which is substantially better than the single-electrode-type batteries the researchers tested. It can also cycle at an extremely high current density of 20 mA/cm2 with a low “overpotential” (that is, the potential difference between a theoretical or thermodynamically determined voltage and the actual voltage under operating conditions) of ~0.5 V without significant signs of short-circuiting, even at a temperature of 55 °C.
Longer lifetime and faster charging
According to the researchers, the new battery technology could make the lifetime of electric vehicles comparable to that of petrol- or diesel-powered ones – 10 to 15 years – without the need to replace the battery. With its high current density, the design could also “pave the way for electric vehicles that can fully charge within 10–20 minutes,” they say.

Beyond the lithium-ion battery
“This proof-of-concept design shows that lithium-metal solid-state batteries could be competitive with commercial lithium-ion batteries,” Li adds. “And the flexibility and versatility of our multilayer design makes it potentially compatible with mass production procedures in the battery industry.”
The researchers say they now plan to scale up their device to a pouch cell the size of an ID card. “We expect that the attractive performance we have presented in our work will hold, since our innovation focuses on the scalable factors of materials chemistry and their combinations,” Li tells Physics World. “The design also opens the door to many new fundamental studies in solid-state battery research that were hitherto not possible without a stable lithium-metal anode.”



